A highly efficient and recyclable ligand-free protocol for the Suzuki coupling reaction of potassium aryltrifluoroborates in water†
Abstract
A highly efficient, recyclable and ligand-free protocol was developed for the Suzuki coupling of aryl halides with potassium aryltrifluoroborates in water using Pd(OAc)2 as a catalyst and Na2CO3 as a base in air. The presence of poly(ethylene glycol) (PEG) was crucial to the efficiency of the protocol. A wide range of functional groups were tolerated under the optimized conditions. Furthermore, the protocol could be extended to the Suzuki coupling of heteroaryl halides with potassium phenyltrifluoroborate, delivering the desired products in moderate to excellent yields. After simple workup, Pd(OAc)2–H2O–PEG could be recycled at least eight times without significant loss in activity.
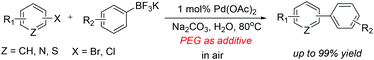

 Please wait while we load your content...
Please wait while we load your content...