Cascade synthesis of quinazolinones from 2-aminobenzonitriles and aryl bromides via palladium-catalyzed carbonylation reaction†
Abstract
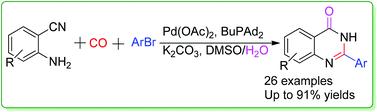
A cascade synthesis of quinazolinones from 2-aminobenzonitriles and aryl bromides through a palladium-catalyzed carbonylation reaction has been developed. Various quinazolinones were produced in moderate to excellent yields. The reactions go through aminocarbonylation of aryl bromides–hydration of nitriles–cyclization sequence. Notably, all the products were isolated by recrystallization.

 Please wait while we load your content...
Please wait while we load your content...