Sunlight, electrochemistry, and sustainable oxidation reactions†
Abstract
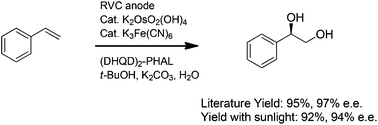
Inexpensive, readily available photovoltaic cells have been used to conduct indirect electrochemical oxidation reactions. The reactions retain the efficiency of the solar-electrochemical method while capitalizing on the unique opportunities for selectivity afforded by a chemical oxidant. The versatility of the electrochemical method allowed for the recycling of Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants all with the same very simple reaction apparatus.

 Please wait while we load your content...
Please wait while we load your content...