Synthesis of high quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream†
Abstract
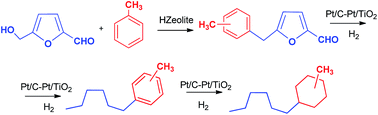
Alkylation of aromatics with HMF is a new route for the synthesis of biofuels. Alkylation of toluene with HMF has been studied in the presence of large pore (HBeta, USY and Mordenite), delaminated zeolites as well as on mesoporous aluminosilicates. In all cases a mixture of monoalkylated products of 5-(o-, m- and p-methyl)benzylfuran-2-carbaldehyde and OBMF coming from self etherification of HMF were obtained. Large pore 3D (USY) and especially 2D (ITQ-2) zeolites are active and selective catalysts for this transformation. The alkylation reaction was extended successfully to other substituted benzenes as well as to a heavy reformate mixture as source aromatic compounds, achieving 91% yield of alkylated products with 93% selectivity. Further hydrodeoxygenation of alkylated compounds in a fixed bed continuous reactor was performed using Pt/C and Pt/TiO2 as catalysts allowing to obtain a hydrocarbon mixture containing alkylcyclohexane compounds that can be used as high quality kerosene.

 Please wait while we load your content...
Please wait while we load your content...