Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques†
Abstract
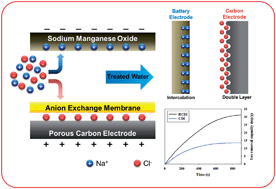
Based on a porous carbon electrode, capacitive deionization (CDI) is a promising desalination technology in which ions are harvested and stored in an electrical double layer. However, the ion removal capacity of CDI systems is not sufficient for desalting high-concentration saline water. Here, we report a novel desalination technique referred to as “hybrid capacitive deionization (HCDI)”, which combines CDI with a battery system. HCDI consists of a sodium manganese oxide (Na4Mn9O18) electrode, an anion exchange membrane, and a porous carbon electrode. In this system, sodium ions are captured by the chemical reaction in the Na4Mn9O18 electrode, whereas chloride ions are adsorbed on the surface of the activated carbon electrode during the desalination process. HCDI exhibited more than double the ion removal sorption capacity (31.2 mg g−1) than a typical CDI system (13.5 mg g−1). Moreover, it was found that the system has a rapid ion removal rate and excellent stability in an aqueous sodium chloride solution. These results thus suggest that the HCDI system could be a feasible method for desalting a highly concentrated sodium chloride solution in capacitive techniques.

 Please wait while we load your content...
Please wait while we load your content...