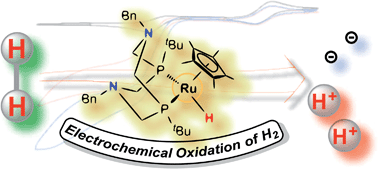
Electrochemical oxidation of H2 catalyzed by ruthenium hydride complexes bearing P2N2 ligands with pendant amines as proton relays†
Abstract
Two Ru hydride complexes, Cp*Ru(PPh2NBn2)H (1-H) and Cp*Ru(PtBu2NBn2)H (2-H) supported by cyclic PR2NR′2 ligands (Cp* = η5-C5Me5; PR2NBn2 = 1,5-dibenzyl,-3,7-R-1,5-diaza-3,7-diphosphacyclooctane, where R = Ph or tBu) have been developed as electrocatalysts for oxidation of H2 (1.0 atm, 22 °C). The turnover frequency of 2-H is 1.2 s−1 at 22 °C (1.0 atm H2) with an overpotential at Ecat/2 of 0.5 V in the presence of exogenous base, DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), while catalysis by 1-H has a turnover frequency of 0.6 s−1 and an overpotential of 0.6 V at Ecat/2. Addition of H2O facilitates oxidation of H2 by 2-H and increases its turnover frequency to 1.9 s−1, while H2O slows down the catalysis by 1-H. In addition, studies of Cp*Ru(dmpm)H (where dmpm = bis(dimethylphosphino)methane), a control complex lacking pendent amines in its diphosphine ligand, confirms the critical roles of the pendant amines of the P2N2 ligands as proton relays in the oxidation of H2.

 Please wait while we load your content...
Please wait while we load your content...