Non-precious metal electrocatalysts with high activity for hydrogen oxidation reaction in alkaline electrolytes†
Abstract
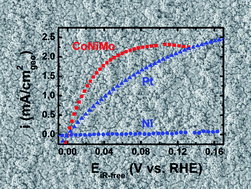
A ternary metallic CoNiMo catalyst is electrochemically deposited on a polycrystalline gold (Au) disk electrode using pulse voltammetry, and characterized for hydrogen oxidation reaction (HOR) activity by temperature-controlled rotating disk electrode measurements in 0.1 M potassium hydroxide (KOH). The catalyst exhibits the highest HOR activity among all non-precious metal catalysts (e.g., 20 fold higher than Ni). At a sufficient loading, the CoNiMo catalyst is expected to outperform Pt and thus provides a promising low cost pathway for alkaline or alkaline membrane fuel cells. Density functional theory (DFT) calculations and parallel H2-temperature programmed desorption (TPD) experiments on structurally much simpler model alloy systems show a trend that CoNiMo has a hydrogen binding energy (HBE) similar to Pt and much lower than Ni, suggesting that the formation of multi-metallic bonds modifies the HBE of Ni and is likely a significant contributing factor for the enhanced HOR activity.

 Please wait while we load your content...
Please wait while we load your content...