Nitrile-assistant eutectic electrolytes for cryogenic operation of lithium ion batteries at fast charges and discharges
Abstract
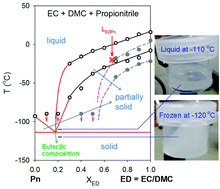
The charge/discharge characteristics of lithium ion batteries at low temperature (LT = −20 °C) are enhanced by using ethylene carbonate (EC)-based electrolytes with the help of assistant solvents of nitriles. Conventional liquid electrolytes (e.g. a mixture of EC and dimethyl carbonate (DMC), abbreviated as LED) cannot support a satisfactory capacity at low temperature as well as at high rates even if electric vehicles require low-temperature operation. Introducing propionitrile or butyronitrile (Pn or Bn) into LED (resulting in LEDPn or LEDBn) as a co-solvent increases significantly the high-rate capacities at −20 °C. For example, LEDPn delivers 62% of the available capacity at 1 C and 46% at 3 C with a 2.7 V cut-off while the control LED provides just 6% and 4% at the same rates. Successful operation at −20 °C with nitrile-assistant electrolytes results from high ionic conductivity, low viscosity and freezing point depression caused by the eutectic behavior of the carbonates (EC/DMC) and Pn. Based on the phase diagram of Pn with EC/DMC, we expect a meaningful battery operation up to −110 °C, probably lower, at the eutectic composition.

 Please wait while we load your content...
Please wait while we load your content...