Avoiding problem reactions at the ferrocenyl-alkyne motif: a convenient synthesis of model, redox-active complexes for molecular electronics†
Abstract
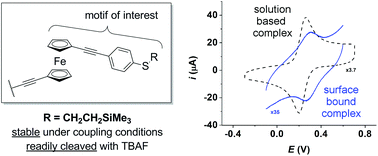
A much improved route to 1,1′-bis(arylethynyl)ferrocenes comprising accessible thiolates on the aryl ring is reported. Unanticipated reactions between AcCl, TBAF–BBr3 and ferrocenyl-alkynes are also discussed, offering a rationale for previous synthetic difficulties.


 Please wait while we load your content...
Please wait while we load your content...