Temperature dependent iodide oxidation by MLCT excited states†
Abstract
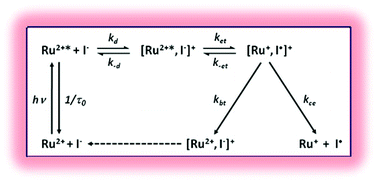
The metal-to-ligand charge transfer (MLCT) excited states of two related heteroleptic Ru(II) compounds [Ru(bpy)2(deeb)]2+ and [Ru(bpy)2(deebq)]2+, where bpy is 2,2′-bipyridine, deeb is 4,4′-(CO2CH2CH3)2-2,2′-bipyridine and deebq is 4,4′-(CO2CH2CH3)2-2,2′-biquinoline, were characterized in fluid acetonitrile by temperature dependent photoluminescence spectroscopies as well as quenching by iodide ions. Photoluminescence emanates from a manifold of thermally equilibrated excited states referred to as the thexi states. Evidence for activated internal conversion to a 4th MLCT excited state was garnered from an Arrhenius analysis of temperature dependent lifetime data. The activation energy was found to be 550 cm−1 for [Ru(bpy)2(deeb)]2+* and 1200 cm−1 for [Ru(bpy)2(deebq)]2+*. The pre-exponential factor abstracted from the Arrhenius analysis of the [Ru(bpy)2(deebq)]2+* data suggested that ligand field excited states might be populated, however there was no evidence for ligand loss photochemistry under the conditions studied. The excited states were found to quench iodide by a dynamic process in good agreement with the Stern–Volmer model. Transient absorption data showed that the quenching mechanism was electron transfer to generate an iodine atom and a reduced ruthenium compound as products. The quenching rate constants abstracted from temperature dependent Stern–Volmer quenching data were corrected for diffusion and activated complex formation to yield electron transfer rate constants that were found to increase markedly with temperature. An Arrhenius analysis of the electron transfer data revealed that electron transfer from iodide to the d-orbitals of the excited state was an activated process with an Ea of 2400 cm−1 for [Ru(bpy)2(deeb)]2+ and 3300 cm−1 for [Ru(bpy)2(deebq)]2+.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...