Synthesis and characterisation of the complete series of B–N analogues of triptycene†
Abstract
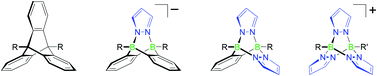
The reaction between the bisborate Li2[o-C6H4(BH3)2] and 2 equivalents of an appropriate pyrazole derivative (HpzR) in the presence of Me3SiCl yields o-phenylene-bridged pyrazaboles HB(μ-pzR)2(μ-o-C6H4)BH (3a–3e; HpzR = 4-iodopyrazole (3a), 4-(trimethylsilyl)pyrazole (3b), 3,5-dimethylpyrazole (3c), 3,5-di(tert-butyl)pyrazole (3d), 3,5-bis(trifluoromethyl)pyrazole (3e)). The synthesis approach thus provides access to uncharged B–N triptycenes bearing (i) functionalisable groups, (ii) electron-donating or -withdrawing substituents and (iii) pyrazole rings of varying steric demand. Treatment of p-R*C6H4BBr2 with the potassium tris(pyrazol-1-yl)borates K[HBpz3] or K[p-R*C6H4Bpz3] yields cationic pyrazolyl-bridged pyrazaboles [p-BrC6H4B(μ-pz)3BH]Br ([4a]Br) and [p-R*C6H4B(μ-pz)3Bp-C6H4R*]Br (R* = Br ([4b]Br), I ([4c]Br), SiMe3 ([4d]Br)), which can be regarded as full B–N analogues of triptycene. The B–H bonds of 3b and [4a]Br are unreactive towards tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH even at temperatures of 80 °C, thereby indicating an appreciable thermal stability of the corresponding B–N cage bonds. Most of the cage compounds are sufficiently inert towards water to allow quick aqueous workup. However, NMR spectroscopy in CD3OD solution reveals degradation of 3b or [4a]Br to the corresponding pyrazoles and o-C6H4(B(OCD3)2)2 or p-BrC6H4B(OCD3)2/B(OCD3)3. The diphenylated species [4b]Br is significantly more stable under the same measurement conditions; even after 76 d, most of the material degrades only to the stage of the syn/anti-pyrazaboles p-BrC6H4(CD3O)B(μ-pz)2B(OCD3)p-C6H4Br (11a/11b). A derivatisation of [4c]Br with nBu3SnC
CH even at temperatures of 80 °C, thereby indicating an appreciable thermal stability of the corresponding B–N cage bonds. Most of the cage compounds are sufficiently inert towards water to allow quick aqueous workup. However, NMR spectroscopy in CD3OD solution reveals degradation of 3b or [4a]Br to the corresponding pyrazoles and o-C6H4(B(OCD3)2)2 or p-BrC6H4B(OCD3)2/B(OCD3)3. The diphenylated species [4b]Br is significantly more stable under the same measurement conditions; even after 76 d, most of the material degrades only to the stage of the syn/anti-pyrazaboles p-BrC6H4(CD3O)B(μ-pz)2B(OCD3)p-C6H4Br (11a/11b). A derivatisation of [4c]Br with nBu3SnC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu through Stille-type coupling reactions furnishes the alkynyl derivative [p-tBuC
CtBu through Stille-type coupling reactions furnishes the alkynyl derivative [p-tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4B(μ-pz)3Bp-C6H4C
CC6H4B(μ-pz)3Bp-C6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu]Br ([4e]Br). Larger B–N aggregates are also accessible: treatment of the tetrakisborate Li4[1,2,4,5-C6H2(BH3)4] with 4 equivalents of HpzR in the presence of Me3SiCl leads to the corresponding B–N pentiptycenes 14a–14d (HpzR = 3,5-bis(trifluoromethyl)pyrazole (14a), 4-(trimethylsilyl)pyrazole (14b), 3,5-dimethylpyrazole (14c), 3,5-di(tert-butyl)pyrazole (14d)).
CtBu]Br ([4e]Br). Larger B–N aggregates are also accessible: treatment of the tetrakisborate Li4[1,2,4,5-C6H2(BH3)4] with 4 equivalents of HpzR in the presence of Me3SiCl leads to the corresponding B–N pentiptycenes 14a–14d (HpzR = 3,5-bis(trifluoromethyl)pyrazole (14a), 4-(trimethylsilyl)pyrazole (14b), 3,5-dimethylpyrazole (14c), 3,5-di(tert-butyl)pyrazole (14d)).

 Please wait while we load your content...
Please wait while we load your content...