Versatile reactivity of a rhodium(i) boryl complex towards ketones and imines†
Abstract
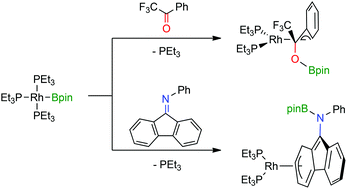
The rhodium(I) boryl complex [Rh(Bpin)(PEt3)3] (1) reacts with the ketones α,α,α-trifluoroacetophenone and 9-fluorenone by insertion of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to give [Rh{η3-C(CF3)(OBpin)C6H5}(PEt3)2] (4) and [Rh{η5-C13H8(OBpin)}(PEt3)2] (6), whereas the reaction with acetophenone leads to the formation of [Rh(H)(PEt3)3] (2), [Rh(OBpin)(PEt3)3] (3) and (E)-(Ph)CH
O bond to give [Rh{η3-C(CF3)(OBpin)C6H5}(PEt3)2] (4) and [Rh{η5-C13H8(OBpin)}(PEt3)2] (6), whereas the reaction with acetophenone leads to the formation of [Rh(H)(PEt3)3] (2), [Rh(OBpin)(PEt3)3] (3) and (E)-(Ph)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHBpin. Treatment of 1 with ketimines generates [Rh{η3-C6H5
CHBpin. Treatment of 1 with ketimines generates [Rh{η3-C6H5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)N(Ph)(Bpin)}(PEt3)2] (7), [Rh{(η3-C12H8)N(Ph)(Bpin)}(PEt3)2] (8) or [Rh{CPh2N(H)(Bpin)}(PEt3)2] (9). The insertion of aldimines into the Rh–B bond gives access to [Rh[η3-CH{N(C6H13)Bpin}C6H5](PEt3)2] (11) or [Rh[η3-CH{N(Ph)Bpin}C6H5](PEt3)2] (12). The latter is converted into the C–H activation product [Rh{(C6H4)-o-N(Bpin)(CH2Ph)}(PEt3)3] (13). Complex 13 reacts with B2pin2 to yield the boryl complex 1 and the amine PhCH2N(Bpin)(C6H4-o-Bpin).
C(Ph)N(Ph)(Bpin)}(PEt3)2] (7), [Rh{(η3-C12H8)N(Ph)(Bpin)}(PEt3)2] (8) or [Rh{CPh2N(H)(Bpin)}(PEt3)2] (9). The insertion of aldimines into the Rh–B bond gives access to [Rh[η3-CH{N(C6H13)Bpin}C6H5](PEt3)2] (11) or [Rh[η3-CH{N(Ph)Bpin}C6H5](PEt3)2] (12). The latter is converted into the C–H activation product [Rh{(C6H4)-o-N(Bpin)(CH2Ph)}(PEt3)3] (13). Complex 13 reacts with B2pin2 to yield the boryl complex 1 and the amine PhCH2N(Bpin)(C6H4-o-Bpin).

 Please wait while we load your content...
Please wait while we load your content...