Thermodynamic stability of La2Mo2−yWyO9, La2Mo2−yWyO8.96+0.02y and La7Mo7(2−y)/2W7y/2O30 (y = 0, 0.5 and 1.0)†
Abstract
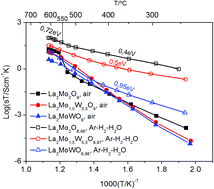
The role of W content on the limit oxygen partial pressure (pO2) for stability of fast oxygen-ion conductors La2Mo2−yWyO9 with y = 0, 0.5 and 1.0 has been studied by means of thermogravimetric analysis (TGA) under controlled atmospheres. At 718 °C, below the pO2 stability limit of La2Mo2−yWyO9, the perovskite related compounds La7Mo7(2−y)/2W7y/2O30 were stabilized even for y = 1.0. At 608 °C, the first stage of reduction of β-La2Mo2−yWyO9 leads to the formation of the crystallized oxygen deficient La2Mo2−yWyO8.6+0.02y phase. X-ray powder diffraction shows that the stabilization of the high temperature β-form through tungsten substitution observed in fully oxidized La2Mo2−yWyO9 samples is preserved upon slight reduction. The n-type conductivity arising from the mixed valence state of molybdenum becomes less and less predominant as the W content increases. Further reduction causes amorphization. At both temperatures, W substitution does not enhance the thermodynamic stability of the La2Mo2−yWyO9 ion conductor under a reducing atmosphere but only slows down the kinetics of reduction.

 Please wait while we load your content...
Please wait while we load your content...