BODIPY functionalized o-carborane dyads for low-energy photosensitization†
Abstract
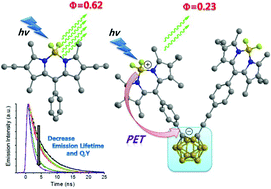
A new type of organic dyad that can induce low-energy photosensitization has been developed; electron donor and electron acceptor units are boron dipyrromethene (BODIPY) and ortho-carborane (o-Cb), respectively. The new dyads consist of a V-shaped BODIPY-(o-Cb)-BODIPY molecular array in which two BODIPY units are substituted onto two adjacent carbon atoms of the central o-Cb. In the presence of the o-Cb unit, as an electron acceptor, significant fluorescence quenching was observed which indicated that photoinduced electron transfer (PET) had occurred from the end-on BODIPY units to the central o-Cb with PET efficiencies of 63–71%. As a result, the corresponding cationic and anionic species that are responsible for the charge transfer state were detected by the serial spectroelectrochemical studies: cationic BODIPY radicals at 400 nm at the applied voltage of 1.44 V and broad absorption bands of anionic o-Cb radicals in the range of 250–490 nm at −1.84 V. Transient absorption studies further confirmed the BODIPY radical anion at 540 nm and the o-Cb radical anion at 350–475 nm with a structureless broad band.

 Please wait while we load your content...
Please wait while we load your content...