Two Strandberg-type organophosphomolybdates: synthesis, crystal structures and catalytic properties†
Abstract
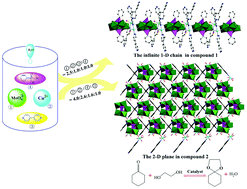
Two novel Strandberg-type organophosphomolybdate hybrid compounds [(Cu(H2O))2(μ-bipy)2(C6H5PO3)2Mo5O15]n (1) and [(Cu(H2O)2)2(μ-bipy)(C6H5PO3)2Mo5O15]n (2) (bipy = 4,4′-bipyridyl) were prepared under mild hydrothermal conditions and structurally characterized by physico-chemical and spectroscopic methods. Single crystal X-ray diffraction analysis reveals that compounds 1 and 2 are polyoxometalate-based Cu-coordination polymers with a three-dimensional framework. In 1, the Cu2+ ions not only link [(C6H5PO3)2Mo5O15]4− (abbreviated as {(C6H5P)2Mo5}) polyanions, but also act as connectors of bipy ligands to produce two symmetrical 1-D chains, all 1-D chains are further held together by polyanions to generate a 3-D network. In 2, each {(C6H5P)2Mo5} polyanion acting as a hexadentate ligand links four Cu(II)-bipy/H2O units, forming 2-D plane structures, which are further bridged by Cu(II)-bipy-Cu(II) fragments to generate a 3-D network. Their fluorescence properties and catalytic properties for the synthesis of cyclohexanone ethylene ketal were also investigated.

 Please wait while we load your content...
Please wait while we load your content...