Phosphine substitution reactions of (η5-cyclopentadienyl)ruthenium bis(triarylphosphine) chloride, CpRu(PAr3)2Cl {PAr3 = PPh3, P(p-CH3C6H4)3, P(p-FC6H4)3, P(p-CH3OC6H4)3, and PPh2(p-CH3C6H4)}: a tale of two mechanisms†
Abstract
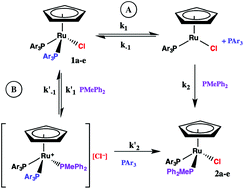
The kinetics of phosphine substitution in CpRu(PAr3)2Cl by PMePh2 under pseudo-first order conditions in CDCl3 have been measured for PAr3 = PPh3, 1a, PPh2(p-tol), 1b, P(p-tol)3, 1c, P(p-CH3OC6H4)3, 1d, and P(p-FC6H4)3), 1e. Activation parameters characteristic of a dissociative pathway (ΔH† = 110–124 ± 2 kJ mol−1, ΔS† = 16–44 ± 5–12 J mol−1 K−1) are observed for all five compounds. The rate of substitution in CpRu(PAr3)2Cl (1a) and CpRu[P(p-FC6H4)3]2Cl (1e) is independent of added chloride ion and decreases in the presence of excess PAr3, however, the rate of substitution in CpRu[P(p-CH3OC6H4)3]2Cl (1d) is first order in added chloride ion and is less dependent on added PAr3. A mechanism involving [CpRu(PAr3)2(PMePh2)]+[Cl]− intermediates contributes to the substitution in 1b–d.

 Please wait while we load your content...
Please wait while we load your content...