Enantiopure N,N,O-scorpionate zinc amide and chloride complexes as efficient initiators for the heteroselective ROP of cyclic esters†
Abstract
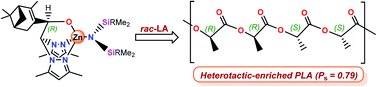
The reaction of bpzbeH, bpzteH (racemic mixture) or (R,R)-bpzmmH (enantiopure) with the amide complexes Zn{N(SiMe3)2}2 or Zn{N(SiHMe2)2}2 in 1 : 1 molar ratio in toluene afforded the mononuclear amide zinc complexes [Zn(NR2)(κ3-NNO)] (1–6) [κ3-NNO = bpzbe, R = SiMe31, SiHMe22; bpzte, R = SiMe33, SiHMe24; (R,R)-bpzmm, SiMe35, SiHMe26]. These complexes were employed in a protonolysis reaction with HCl–Et2O in 2 : 1 molar ratio to yield the dinuclear amide/chloride zinc complexes [Zn(κ2-NN-μ-O)2{ZnCl(NR2)}] (7–12) [κ2-NN-μ-O = bpzbe, R = SiMe37, SiHMe28; bpzte, R = SiMe39, SiHMe210; (R,R)-bpzmm, SiMe311, SiHMe212]. The mononuclear complexes 5 and 6 and dinuclear complexes 11 and 12 are the first enantiopure-scorpionate zinc amide complexes to be synthesized. The single-crystal X-ray structure analysis of derivatives 1 and 3 confirmed a monomeric 4-coordinative structure in which the heteroscorpionate ligands are in a κ3 coordination mode, while 8 had a dimeric molecular disposition with two μ-bridging alkoxides of the heteroscorpionate ligands between the two six- and four-coordinate Zn(II) centers. Interestingly, the chiral amide-containing zinc complexes 1–5 and 11 can act as single-component initiators for the ring-opening polymerization of ε-caprolactone and lactides under mild conditions, affording, in a few hours, medium/low molecular weight polymers with low polydispersity indices. MALDI-ToF mass spectra confirmed that the initiation occurred through a nucleophilic attack by the amide on the lactide monomer, and inspection of the kinetic parameters showed that propagations present the usual pseudo-first order dependence on monomer and catalyst concentrations. In addition, microstructural analysis of poly(rac-lactide)s revealed that the myrtenal substituent on the alkoxide fragment has a significant influence on the degree of stereoselectivity, producing enriched-heterotactic PLAs with a Ps value of up to 0.79 under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...