A computational study of the mechanism for water oxidation by (bpc)(bpy)RuIIOH2†
Abstract
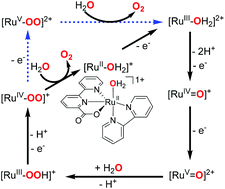
A mechanistic study on the catalytic cycle water oxidation with 1 [(bpc)(bpy)RuIIOH2]+ (Hbpc = 2,2′-bipyridine-6-carboxylic acid, bpy = 2,2′-bipyridine) is described in this paper. Stepwise oxidation via proton-coupled electron transfer gives 3 [(bpc)(bpy)RuIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]+. An active 4 [(bpc)(bpy)RuV
O]+. An active 4 [(bpc)(bpy)RuV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]2+, which is involved in the OO bond formation is generated from further 1e− oxidation of 3. Another different possible reaction at 4 was investigated and new destructive paths involving overoxidation of the metal were identified. The most viable path for OO bond formation via a water nucleophilic attack at the oxo of 4 is found to be the rate-determining step in this water oxidation catalytic cycle, and the hydro-peroxo 6 [(bpc)(bpy)RuIIIOOH]+ is generated accompanied with a proton transfer. The super-oxo 7side-on [(bpc)(bpy)RuIVOO]+ and 8side-on [(bpc)(bpy)RuVOO]2+, both low spin species, are generated by further oxidations of 6. Through an intersystem crossing they can transform to their high spin states, 9end-on [(bpc)(bpy)RuIVOO]+ and 12end-on [(bpc)(bpy)RuVOO]2+, respectively. Following a dissociative pathway O2 is readily generated from both 9end-on and 12end-on.
O]2+, which is involved in the OO bond formation is generated from further 1e− oxidation of 3. Another different possible reaction at 4 was investigated and new destructive paths involving overoxidation of the metal were identified. The most viable path for OO bond formation via a water nucleophilic attack at the oxo of 4 is found to be the rate-determining step in this water oxidation catalytic cycle, and the hydro-peroxo 6 [(bpc)(bpy)RuIIIOOH]+ is generated accompanied with a proton transfer. The super-oxo 7side-on [(bpc)(bpy)RuIVOO]+ and 8side-on [(bpc)(bpy)RuVOO]2+, both low spin species, are generated by further oxidations of 6. Through an intersystem crossing they can transform to their high spin states, 9end-on [(bpc)(bpy)RuIVOO]+ and 12end-on [(bpc)(bpy)RuVOO]2+, respectively. Following a dissociative pathway O2 is readily generated from both 9end-on and 12end-on.

 Please wait while we load your content...
Please wait while we load your content...