Metal-mediated coupling of amino acid esters with isocyanides leading to new chiral acyclic aminocarbene complexes†
Abstract
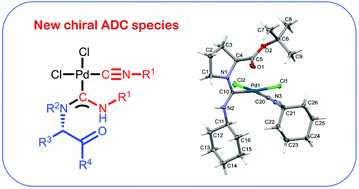
Metal-mediated coupling between equimolar amounts of cis-[PdCl2(CNR1)2] (1–5) and the amino acid esters L-HTyrOMe (7) or L-HProOtBu (8) proceeds at 40 °C in chloroform over ca. 6 h. The subsequent workup affords the complexes cis-[PdCl2(CNR1){C(TyrOMe)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHR1}] (R1 = Xyl 9, 2-Cl-6-Me-C6H310) or cis-[PdCl2(CNR1){C(ProOtBu)
NHR1}] (R1 = Xyl 9, 2-Cl-6-Me-C6H310) or cis-[PdCl2(CNR1){C(ProOtBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHR1}] (R1 = Xyl 11, 2-Cl-6-Me-C6H312, Cy 13, tBu 14, 2-naphthyl 15) in good to excellent isolated yields (75–94%). The corresponding reaction between trans-[PdI2(CNR1)2] (6) and 8 brings about the formation of trans-[PdI2(CNCy){C(ProOtBu)
NHR1}] (R1 = Xyl 11, 2-Cl-6-Me-C6H312, Cy 13, tBu 14, 2-naphthyl 15) in good to excellent isolated yields (75–94%). The corresponding reaction between trans-[PdI2(CNR1)2] (6) and 8 brings about the formation of trans-[PdI2(CNCy){C(ProOtBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHCy}] (16, 76% isolated yield). The reaction of 6 with 7 proceeds non-selectively giving a broad mixture of products. Complexes 9–16 were characterized by elemental analyses (C, H, N), ESI+/−-MS, IR, 1D (1H, 13C{H}) and 2D (1H,1H-COSY, 1H,13C-HMQC/1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopic techniques, and by single-crystal X-ray diffraction (for 9, 11–13, and 16).
NHCy}] (16, 76% isolated yield). The reaction of 6 with 7 proceeds non-selectively giving a broad mixture of products. Complexes 9–16 were characterized by elemental analyses (C, H, N), ESI+/−-MS, IR, 1D (1H, 13C{H}) and 2D (1H,1H-COSY, 1H,13C-HMQC/1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopic techniques, and by single-crystal X-ray diffraction (for 9, 11–13, and 16).

 Please wait while we load your content...
Please wait while we load your content...