Computed ligand effects on the oxidative addition of phenyl halides to phosphine supported palladium(0) catalysts†
Abstract
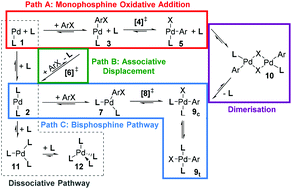
The manifold of reaction pathways for the oxidative addition of phenyl bromide and phenyl chloride substrates to phosphine-modified palladium(0) complexes has been investigated with dispersion-corrected density functional theory (B3LYP-D2) for a range of synthetically relevant ligands, permitting the evaluation of ligand, substrate and method effects on calculated predictions. Bulky and electron-rich ligands PtBu3 and SPhos can access low-coordinate complexes more easily, facilitating formation of the catalytically active species throughout the cycle. While the bisphosphine oxidative addition step is reasonably facile for the smaller PCy3 and PPh3 ligands, the dissociation of these ligands to generate reactive palladium complexes becomes more important and the catalyst is more likely to become trapped in unreactive intermediates. This study demonstrates the feasibility of exploring the catalytic manifold for synthetically relevant ligands with computational chemistry, but also highlights the remaining challenges.

 Please wait while we load your content...
Please wait while we load your content...