Electron transfer dynamics and excited state branching in a charge-transfer platinum(ii) donor–bridge-acceptor assembly†
Abstract
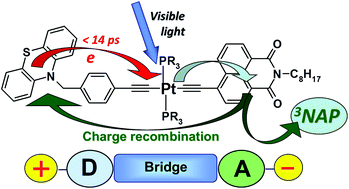
A linear asymmetric Pt(II) trans-acetylide donor–bridge-acceptor triad designed for efficient charge separation, NAP![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Pt(PBu3)2
Pt(PBu3)2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ph–CH2–PTZ (1), containing strong electron acceptor and donor groups, 4-ethynyl-N-octyl-1,8-naphthalimide (NAP) and phenothiazine (PTZ) respectively, has been synthesised and its photoinduced charge transfer processes characterised in detail. Excitation with 400 nm, ∼50 fs laser pulse initially populates a charge transfer manifold stemming from electron transfer from the Pt-acetylide centre to the NAP acceptor and triggers a cascade of charge and energy transfer events. A combination of ultrafast time-resolved infrared (TRIR) and transient absorption (TA) spectroscopies, supported by UV-Vis/IR spectroelectrochemistry, emission spectroscopy and DFT calculations reveals a self-consistent photophysical picture of the excited state evolution from femto- to milliseconds. The characteristic features of the NAP-anion and PTZ-cation are clearly observed in both the TRIR and TA spectra, confirming the occurrence of electron transfer and allowing the rate constants of individual ET-steps to be obtained. Intriguingly, 1 has three separate ultrafast electron transfer pathways from a non-thermalised charge transfer manifold directly observed by TRIR on timescales ranging from 0.2 to 14 ps: charge recombination to form either the intraligand triplet 3NAP with 57% yield, or the ground state, and forward electron transfer to form the full charge-separated state 3CSS (3[PTZ+–NAP−]) with 10% yield as determined by target analysis. The 3CSS decays by charge-recombination to the ground state with ∼1 ns lifetime. The lowest excited state is 3NAP, which possesses a long lifetime of 190 μs and efficiently sensitises singlet oxygen. Overall, molecular donor–bridge-acceptor triad 1 demonstrates excited state branching over 3 different pathways, including formation of a long-distant (18 Å) full charge-separated excited state from a directly observed vibrationally hot precursor state.
Ph–CH2–PTZ (1), containing strong electron acceptor and donor groups, 4-ethynyl-N-octyl-1,8-naphthalimide (NAP) and phenothiazine (PTZ) respectively, has been synthesised and its photoinduced charge transfer processes characterised in detail. Excitation with 400 nm, ∼50 fs laser pulse initially populates a charge transfer manifold stemming from electron transfer from the Pt-acetylide centre to the NAP acceptor and triggers a cascade of charge and energy transfer events. A combination of ultrafast time-resolved infrared (TRIR) and transient absorption (TA) spectroscopies, supported by UV-Vis/IR spectroelectrochemistry, emission spectroscopy and DFT calculations reveals a self-consistent photophysical picture of the excited state evolution from femto- to milliseconds. The characteristic features of the NAP-anion and PTZ-cation are clearly observed in both the TRIR and TA spectra, confirming the occurrence of electron transfer and allowing the rate constants of individual ET-steps to be obtained. Intriguingly, 1 has three separate ultrafast electron transfer pathways from a non-thermalised charge transfer manifold directly observed by TRIR on timescales ranging from 0.2 to 14 ps: charge recombination to form either the intraligand triplet 3NAP with 57% yield, or the ground state, and forward electron transfer to form the full charge-separated state 3CSS (3[PTZ+–NAP−]) with 10% yield as determined by target analysis. The 3CSS decays by charge-recombination to the ground state with ∼1 ns lifetime. The lowest excited state is 3NAP, which possesses a long lifetime of 190 μs and efficiently sensitises singlet oxygen. Overall, molecular donor–bridge-acceptor triad 1 demonstrates excited state branching over 3 different pathways, including formation of a long-distant (18 Å) full charge-separated excited state from a directly observed vibrationally hot precursor state.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States


 Please wait while we load your content...
Please wait while we load your content...