NiII, CuII and ZnII complexes with a sterically hindered scorpionate ligand (TpmsPh) and catalytic application in the diasteroselective nitroaldol (Henry) reaction†
Abstract
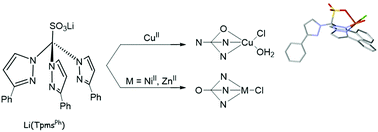
The NiII and ZnII complexes [MCl(TpmsPh)] (TpmsPh = SO3C(pzPh)3, pz = pyrazolyl; M = Ni 2 or Zn 3) and the CuII complex [CuCl(TpmsPh)(H2O)] (4) have been prepared by treatment of the lithium salt of the sterically demanding and coordination flexible tris(3-phenyl-1-pyrazolyl)methanesulfonate (TpmsPh)− (1) with the respective metal chlorides. The (TpmsPh)− ligand shows the N3 or N2O coordination modes in 2 and 3 or in 4, respectively. Upon reaction of 2 and 3 with Ag(CF3SO3) in acetonitrile the complexes [M(TpmsPh)(MeCN)](CF3SO3) (M = Ni 5 or Zn 6, respectively) were formed. The compounds were obtained in good yields and characterized by analytic and spectral (IR, 1H and 13C{1H} NMR, ESI-MS) data, density functional theory (DFT) methods and {for 4 and [nBu4N](TpmsPh) (7), the latter obtained upon Li+ replacement by [nBu4N]+ in Li(TpmsPh)} by single crystal X-ray diffraction analysis. The ZnII and CuII complexes (3 and 4, respectively) act as efficient catalyst precursors for the diastereoselective nitroaldol reaction of benzaldehydes and nitroethane to the corresponding β-nitroalkanols (up to 99% yield, at room temperature) with diastereoselectivity towards the formation of the anti isomer, whereas the NiII complex 2 only shows a modest catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...