Syntheses of sterically shielded stable carbenes of the 1,2,4-triazole series and their corresponding palladium complexes: efficient catalysts for chloroarene hydrodechlorination†
Abstract
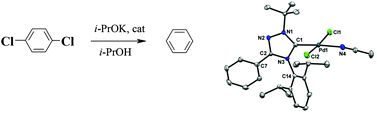
The new sterically shielded 1,3,4-trisubstituted 1,2,4-triazol-5-ylidenes 8b–d were synthesized by a three step method starting from 2-phenyl-1,3,4-oxadiazole. The syntheses of palladium complexes 9a–d and 10a–d (including the sterically shielded derivatives 9c,d and 10a–d) were carried out via the reactions of the stable carbenes 8a–d with palladium halogenide salts in THF or toluene solution. Complexes 9c,d and 10a–d were found to be excellent catalysts for the reductive dechlorination (hydrodechlorination) of p-dichlorobenzene. The structures of 8c, 9a,b, and 10a were determined by single-crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...