A Eu2+ and Mn2+-coactivated fluoro-apatite-structure Ca6Y2Na2(PO4)6F2 as a standard white-emitting phosphor via energy transfer
Abstract
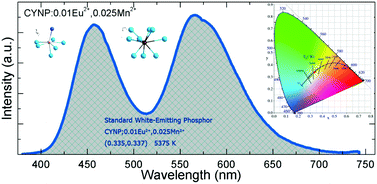
In this study, we synthesized and characterized a series of Eu2+ and Mn2+-coactivated fluoro-apatite-structure Ca6Y2Na2(PO4)6F2 phosphors, which can provide high-quality standard white emission from a single-phase host. The powder X-ray diffraction, photoluminescence spectra, fluorescence decay curves, chromaticity coordinates, and correlated color temperature of the obtained phosphors were measured and are discussed in detail. Energy transfer from the Eu2+ to the Mn2+ ions was demonstrated by the overlap between the emission spectrum of the Eu2+ ions and the excitation spectrum of the Mn2+ ions, and the systematic relative decline and growth of the emission bands of Eu2+ and Mn2+, respectively. A possible energy transfer mechanism was proposed based on the experimental results and analysis. It was discovered that the emission colors of the phosphor varied from blue through white and eventually to yellow by properly tuning the doping content of Mn2+ with a fixed Eu2+ content through the principle of energy transfer. The developed phosphors can be efficiently excited in the UV region and exhibit white-light emission, making them attractive as white-emitting conversion phosphors for UV-based white light emitting diodes.

 Please wait while we load your content...
Please wait while we load your content...