Vanadium complexes with multidentate amine bisphenols†
Abstract
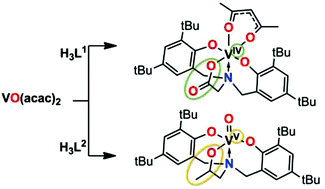
The reaction of VO(acac)2 (acac− = acetyl acetonate) with tripodal glycine bisphenol H3L1 under an ambient atmosphere yields a hexacoordinated vanadium(IV) complex [V(acac)(L1)] (1). The corresponding reactions with tripodal 2-propanolamine bisphenol H3L2 and potentially pentadentate ethoxyethanolamine bisphenol H3L3 lead to the oxidation of the metal centre and formation of mononuclear oxovanadium(V) complexes [VO(L2)] (2) and [VO(L3)] (3), respectively. Alternatively, these latter two complexes can be prepared using VOSO4·5H2O or VO(OPr)3 as a precursor. The CV of 1 in an ACN solution shows a reversible one-electron process at E1/2 = +1.18 V, whereas 2 and 3 have an irreversible redox response at −1.6 V and −1.2 V, respectively. Complexes 2 and 3 show moderate activity in the epoxidation of cis-cyclooctene by tert-BuOOH at 50 °C.

 Please wait while we load your content...
Please wait while we load your content...