Carbazole-BODIPY conjugates: design, synthesis, structure and properties†
Abstract
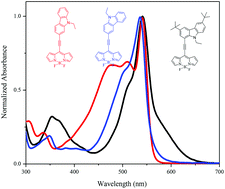
A set of carbazole substituted BODIPYs 2a–2c were designed and synthesized by the Pd-catalysed Sonogashira cross-coupling reaction. The effects of variation in the donor strength of various carbazoles were investigated by photophysical, electrochemical and computational studies. The electronic absorption spectra of BODIPYs 2a and 2c show charge transfer bands, which show red shift in polar solvents. The BODIPYs 2a–2c are highly fluorescent in nonpolar solvents (emission from the localized state) and poorly fluorescent in polar solvents (emission from the charge transfer state). The photophysical and electrochemical studies reveal strong donor–acceptor interaction between carbazole and BODIPY and follows the order 2a > 2c > 2b. The computational calculations show good agreement with the experimental results. The single crystal structures of BODIPYs 2a–2c are reported, which exhibit interesting supramolecular interactions. The packing diagrams of 2a show a zigzag 3D structural arrangement, whereas 2b and 2c show complex 3D structural motifs.

 Please wait while we load your content...
Please wait while we load your content...