Two coordination polymers constructed from a multidentate carboxylic acid ligand with a tertiary amine serve as acid–base catalysts for the synthesis of chloropropene carbonate from CO2 under atmospheric pressure†
Abstract
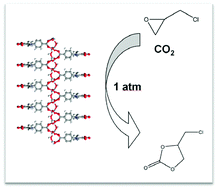
Two new coordination polymers, [Ni(H2O)(Hpdcd)(H2O)2]·DMF (1) and [Co(H2O)(Hpdcd)(H2O)2]·DMF (2) (H3pdcd = 1-(4-carboxyphenyl)-2,5-dimethyl, 1H-pyrrole-3,4-dicarboxylic acid), which were designed based on a tertiary amine ligand, were synthesized and characterized using multiple spectroscopy techniques, including single-crystal X-ray diffraction. These two 1D linear chains possess the properties of both a Lewis acid and organic base, which was confirmed by temperature programmed desorption of ammonia and on-line mass spectrometry (NH3-TPD-MS), and selective sorption for carbon dioxide. Due to their acid–base properties, the compounds exhibited high catalytic activity, in the absence of co-catalysts, for solvent-free synthesis of chloropropene carbonate from CO2 and epichlorohydrin under atmospheric CO2 pressure. The yields of chloropropene carbonate were 88% and 87% for 1 and 2, respectively, under the optimized conditions.

 Please wait while we load your content...
Please wait while we load your content...