Palladium dichloride adduct of N,N-bis-(diphenylphosphanylmethyl)-2-aminopyridine: synthesis, structure and catalytic performance in the decarboxylative cross-coupling of 4-picolinic acid with aryl bromide†
Abstract
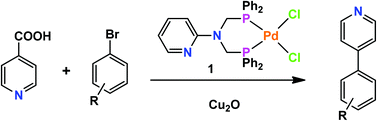
Reaction of PdCl2 with N,N-bis-(diphenylphosphanylmethyl)-2-aminopyridine (bdppmapy) afforded a mononuclear complex [(bdppmapy)PdCl2] (1). Compound 1 was characterized by elemental analysis, IR, 1H, 13C and 31P NMR, electrospray ion mass spectra (ESI-MS) and X-ray single crystal crystallography. The Pd(II) center in 1 is chelated by bdppmapy, showing a cis-square planar geometry. With the assistance of additive Cu2O, complex 1 exhibited good catalytic activity toward the decarboxylative cross-coupling reactions between 4-picolinic acid and aryl bromides. In the presence of only 2 mol% catalyst, a family of 4-aryl-pyridines could be isolated in up to 83% yield.

 Please wait while we load your content...
Please wait while we load your content...