Reduction of C,N-chelated chloroborane: straightforward formation of the unprecedented 1H-2,1-benzazaborolyl potassium salt†
Abstract
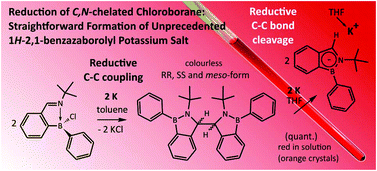
Reduction of C,N-chelated chloroborane [2-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NtBu)C6H4]BPhCl (1) with the potassium metal afforded (3,3′)-bis(1-Ph-2-tBu-1H-2,1-benzazaborole) (2). Compound 2 is formed via C–C reductive coupling reaction. Subsequent reduction of 2 with two equivalents of the potassium metal produced orange crystals of 1Ph-2tBu-1H-2,1-benzazaborolyl (Bab) potassium salt K(THF)(Bab) (3). Compound 3 is able to react with simple electrophiles (MeI or Me3SiCl) resulting in the formation of substituted 1H-2,1-benzazaboroles.
NtBu)C6H4]BPhCl (1) with the potassium metal afforded (3,3′)-bis(1-Ph-2-tBu-1H-2,1-benzazaborole) (2). Compound 2 is formed via C–C reductive coupling reaction. Subsequent reduction of 2 with two equivalents of the potassium metal produced orange crystals of 1Ph-2tBu-1H-2,1-benzazaborolyl (Bab) potassium salt K(THF)(Bab) (3). Compound 3 is able to react with simple electrophiles (MeI or Me3SiCl) resulting in the formation of substituted 1H-2,1-benzazaboroles.

 Please wait while we load your content...
Please wait while we load your content...