Ca3Be6B5O16F: the first alkaline-earth beryllium borate with fluorine anions†
Abstract
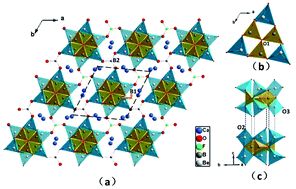
The first all-alkaline-earth beryllium borate with fluorine anions, Ca3Be6B5O16F, was synthesized by a spontaneous crystallization flux method using LiF–B2O3 as the flux. The structural framework of Ca3Be6B5O16F is composed of the inter-connected [Be6B3O16] and [BO3] fundamental building blocks, with [CaO7F] distorted polyhedra located in the interstitial sites. The [Be6B3O16] group is discovered for the first time in beryllium borates. The UV-Vis-NIR diffuse-reflectance spectrum demonstrates that its UV cutoff edge is below 200 nm, and this is confirmed by first-principles studies. Thermal analysis reveals an incongruent feature at 1321 K. IR spectroscopy measurements are consistent with the crystallographic study. These data reveal that the crystal could have an application as a deep-ultraviolet optical material.

 Please wait while we load your content...
Please wait while we load your content...