Structural and thermodynamic similarities of phases in the Li–Tt (Tt = Si, Ge) systems: redetermination of the lithium-rich side of the Li–Ge phase diagram and crystal structures of Li17Si4.0−xGex for x = 2.3, 3.1, 3.5, and 4 as well as Li4.1Ge†
Abstract
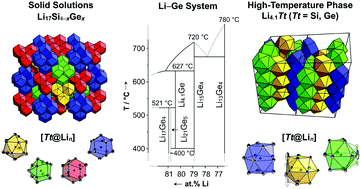
A reinvestigation of the lithium-rich section of the Li–Ge phase diagram reveals the existence of two new phases, Li17Ge4 and Li4.10Ge (Li16.38Ge4). Their structures are determined by X-ray diffraction experiments of large single crystals obtained from equilibrated melts with compositions Li95Ge5 and Li85Ge15. Excess melt is subsequently removed through isothermal centrifugation at 400 °C and 530 °C, respectively. Li17Ge4 crystallizes in the space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (a = 18.8521(3) Å, V = 6700.1(2) Å3, Z = 20, T = 298 K) and Li4.10Ge (Li16.38Ge4) in Cmcm (a = 4.5511(2) Å, b = 22.0862(7) Å, c = 13.2751(4) Å, V = 1334.37(8) Å3, Z = 16, T = 123 K). Both phases are isotypic with their Si counterparts and are further representative of the Li17Pb4 and Li4.11Si structure types. Additionally, the solid solutions Li17Si4−xGex follows Vegard's law. A comparison of the GeLin coordination polyhedra shows that isolated Ge atoms are 13- and 14-coordinated in Li17Ge4, whereas in Li16.38Ge4 the Ge atoms possess coordination numbers 12 and 13. Regarding the thermodynamic stability, Li16.38Ge4 is assigned a high-temperature phase existing between ∼400 °C and 627 °C, whereas Li17Ge4 decomposes peritectically at 520–522 °C. Additionally, the decomposition of Li16.38Ge4 below ∼400 °C was found to be very sluggish. These findings are manifested by differential scanning calorimetry, long-term annealing experiments and the results from melt equilibration experiments. Interestingly, the thermodynamic properties of the lithium-rich tetrelides Li17Tt4 and Li4.1Tt (Li16.4Tt4) are very similar (Tt = Si, Ge). Besides Li15Tt4, Li14Tt6, Li12Tt7, and LiTt, the title compounds are further examples of isotypic tetrelides in the systems Li–Tt.
3m (a = 18.8521(3) Å, V = 6700.1(2) Å3, Z = 20, T = 298 K) and Li4.10Ge (Li16.38Ge4) in Cmcm (a = 4.5511(2) Å, b = 22.0862(7) Å, c = 13.2751(4) Å, V = 1334.37(8) Å3, Z = 16, T = 123 K). Both phases are isotypic with their Si counterparts and are further representative of the Li17Pb4 and Li4.11Si structure types. Additionally, the solid solutions Li17Si4−xGex follows Vegard's law. A comparison of the GeLin coordination polyhedra shows that isolated Ge atoms are 13- and 14-coordinated in Li17Ge4, whereas in Li16.38Ge4 the Ge atoms possess coordination numbers 12 and 13. Regarding the thermodynamic stability, Li16.38Ge4 is assigned a high-temperature phase existing between ∼400 °C and 627 °C, whereas Li17Ge4 decomposes peritectically at 520–522 °C. Additionally, the decomposition of Li16.38Ge4 below ∼400 °C was found to be very sluggish. These findings are manifested by differential scanning calorimetry, long-term annealing experiments and the results from melt equilibration experiments. Interestingly, the thermodynamic properties of the lithium-rich tetrelides Li17Tt4 and Li4.1Tt (Li16.4Tt4) are very similar (Tt = Si, Ge). Besides Li15Tt4, Li14Tt6, Li12Tt7, and LiTt, the title compounds are further examples of isotypic tetrelides in the systems Li–Tt.
- This article is part of the themed collection: Inorganic Chemistry for Renewable Energy Conversion and Storage

 Please wait while we load your content...
Please wait while we load your content...