Rational development of iron catalysts for asymmetric transfer hydrogenation
Abstract
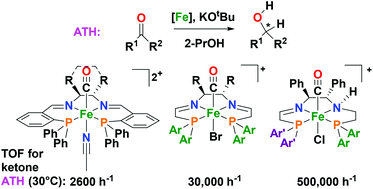
The asymmetric reduction of ketones and imines by transfer of hydrogen from isopropanol as the solvent catalyzed by metal complexes is a very useful method for preparing valuable enantioenriched alcohols and amines. Described here is the development of three generations of progressively more active iron catalysts for this transformation. Key features of this process of discovery involved the realization that one carbonyl ligand was needed (as in hydrogenases), the synthesis of modular ligands templated by iron, the elucidation of the mechanisms of catalyst activation and action, as well as the rational synthesis of precursors that lead directly and easily to the species in the catalytic cycle. The discovery that iron, an abundant element that is essential to life, can form catalysts of these hydrogenation reactions is a contribution to green chemistry.

 Please wait while we load your content...
Please wait while we load your content...