Alkaline earth metal-based metal–organic framework: hydrothermal synthesis, X-ray structure and heterogeneously catalyzed Claisen–Schmidt reaction†
Abstract
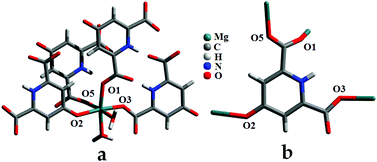
Two alkaline earth metal-based carboxylate systems, [Mg(HL)(H2O)2]n (1) and [Ca(H2L)2]n (2) (H3L = chelidamic acid) have been hydrothermally synthesized, and characterized by single-crystal X-ray diffraction, IR, elemental analysis, and thermo-gravimetric analysis. Compound 1 has a 2D structure incorporating two water molecules. The dehydrated species, 1a, generated from 1 by removal of the coordinated water, has been characterized by thermo-gravimetric analysis, IR, elemental analysis and variable temperature powder X-ray diffraction. Both 1 and its dehydrated species 1a catalyze the Claisen–Schmidt reaction under heterogeneous conditions, but 1a is a more effective catalyst under environmentally friendly conditions. The catalyst can readily be recovered and reused in successive cycles without detectable loss of activity. Compound 2 has a 3D structure and is thermally stable up to 540 °C, but is inactive catalytically.

 Please wait while we load your content...
Please wait while we load your content...