Synthesis and structure of a ferric complex of 2,6-di(1H-pyrazol-3-yl)pyridine and its excellent performance in the redox-controlled living ring-opening polymerization of ε-caprolactone†
Abstract
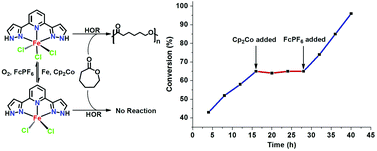
The reaction of FeCl3 with a pincer ligand, 2,6-di(1H-pyrazol-3-yl)pyridine (bppyH2), produced a mononuclear Fe(III) complex [Fe(bppyH2)Cl3] (1), which could be reduced to the corresponding Fe(II) dichloride complex [Fe(bppyH2)Cl2] (2) by suitable reducing agents such as Cp2Co or Fe powder. 1 and 2 exhibited a reversible transformation from each other with appropriate redox reagents. 1 could be utilized as a pre-catalyst to initiate the ring-opening polymerization of ε-caprolactone in the presence of alcohol but 2 did not work. The 1/alcohol system displayed characteristics of a well-controlled polymerization with the resulting poly(ε-caprolactone) having low molecular weight distributions, a linear tendency of molecular weight evolution with conversion, and polymer growth observed for the sequential additions of ε-caprolactone monomer to the polymerization reaction. The polymerization was completely turned off by the in situ reduction of the catalytic Fe center via Cp2Co and then turned back upon the addition of [Cp2Fe]PF6. The rate of polymerization was modified by switching in situ between the Fe(III) and Fe(II) species.

 Please wait while we load your content...
Please wait while we load your content...