Structural characterization and biological evaluation of a clioquinol–ruthenium complex with copper-independent antileukaemic activity†
Abstract
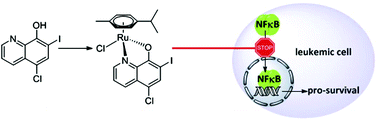
In this study, we present the synthesis, biological characterization, and first crystal structure of an organometallic–clioquinol complex. Combining ruthenium with the established apoptotic agent and 8-hydroxyquinoline derivative, clioquinol, resulted in a complex that induces caspase-dependent cell death in leukaemia cells. This activity is copper independent and is improved compared to the parent compound, clioquinol. The study of the mode of action reveals that this clioquinol–ruthenium complex does not intercalate between DNA base pairs. Additionally, this clioquinol–ruthenium complex shows proteasome-independent inhibition of the NFκB signalling pathway, with no effects on cell-cycle distribution. These data suggest a mechanism of action that involves a target profile that is different from that for clioquinol alone.

 Please wait while we load your content...
Please wait while we load your content...