Tetraphosphonated thiophene ligand: mixing the soft and the hard†
Abstract
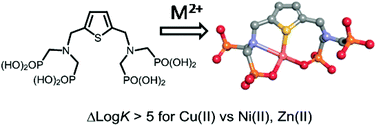
The synthesis of ligand LTH8, based on a thiophene framework containing two bis(aminomethyldiphosphonate) functions in the ortho position to the central sulfur atom, is described, together with the characterization of the intermediate compounds. The physico-chemical properties of the ligand were first studied by means of potentiometry and UV-Vis absorption spectrophotometric titrations to determine its pK values. Six successive equilibrium constants were determined in aqueous solutions. The same means were then used to quantify the interactions of the ligand with Ni(II), Cu(II) and Zn(II). Following the conventional Irving–Williams trend, LT was shown to have the highest affinity towards Cu(II) (log K(CuLT) = 16.11(3)), while Zn(II) and Ni(II) showed similar values (log K(MLT) = 10.81(8) and 10.9(1), respectively), revealing a large selectivity of LT toward Cu(II). Based on a combination of UV-Vis absorption spectroscopy and EPR measurements as a function of pH, along with DFT calculations, the coordination behavior of the hard phosphonate, medium amino and soft thiophene entities are questioned regarding their coordination to the Cu atom.

 Please wait while we load your content...
Please wait while we load your content...