Multiplying the electron storage capacity of a bis-tetrazine pincer ligand†
Abstract
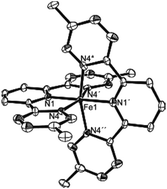
An unexpected doubling in redox storage emerging from a new pincer ligand upon bis-ligation of iron(II) is described. When tetrazine arms are present at the two ortho positions of pyridine, the resulting bis-tetrazinyl pyridine (btzp) pincer ligand displays a single one-electron reduction at ca. −0.85 V vs. Ag/AgCl. Complexation to iron, giving the cation Fe(btzp)22+, shows no oxidation but four reduction waves in cyclic voltammetry instead of the two expected for the two constituent ligands. Mossbauer, X-ray diffraction and NMR studies show the iron species to contain low spin Fe(II), but with evidence of back donation from iron to the pincer ligands. CV and UV-Vis spectroelectrochemistry, as well as titration studies as monitored by CV, electronic spectra and EPR reveal the chemical reversibility of forming the reduced species. DFT and EPR studies show varying degrees of delocalization of unpaired spin in different species, including that of a btzp−1 radical anion, partnered with various cations.

 Please wait while we load your content...
Please wait while we load your content...