Ferro- and antiferromagnetic coupling in a chlorido-bridged, tetranuclear Cu(ii) complex†
Abstract
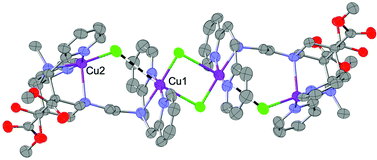
A bispidine-like ligand involving four pyridine-N and three aliphatic-N donor atoms forms a bimetallic species with CuCl2 in which all seven N-donors are bound and which aggregates in the crystal through double chloride-bridging to give a tetranuclear unit. The magnetism of this solid can be interpreted in terms of a relatively weak antiferromagnetic coupling between the two Cu(II) centres of the dinuclear subunits and a strong ferromagnetic coupling of the Cu(II) centres in different dinuclear units involved in the bis-chlorido bridge. In solution, the assembly decays into the dinuclear subunits and, in agreement with the solid state studies, the interaction between the corresponding CuII centres is shown to be primarily due to dipole–dipole coupling.

 Please wait while we load your content...
Please wait while we load your content...