Zinc metal–organic frameworks: efficient catalysts for the diastereoselective Henry reaction and transesterification†
Abstract
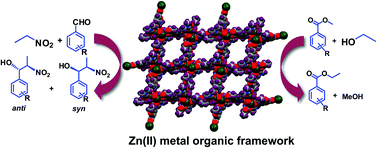
Three new compounds bearing different flexible side functional groups, viz. 2-acetamidoterephthalic acid (H2L1), 2-propionamidoterephthalic acid (H2L2) and 2-benzamidoterephthalic acid (H2L3), were synthesized and their coordination reactions with zinc(II) were studied. X-ray crystallography showed the formation of novel metal organic frameworks with different dimensionalities, where the side functional groups of amidoterephthalic acid and/or auxiliary ligands were found to play significant roles. These frameworks [Zn2(L1)2(4,4′-bipyridine)2(H2O)(DMF)]n (1), [Zn4(L2)3(OH)2(DMF)2(H2O)2]n (2) and [Zn(L3)(H2O)2]n·n/2(1,4-dioxane) (3) act as heterogeneous polymeric solid catalysts not only for the diastereoselective nitroaldol (Henry) reaction of different aldehydes with nitroalkanes but also for transesterification reactions. These MOF-based heterogeneous catalysts can be recycled without losing their activity.

 Please wait while we load your content...
Please wait while we load your content...