The structural diversity and photoluminescent properties of cadmium thiophenedicarboxylate coordination polymers†
Abstract
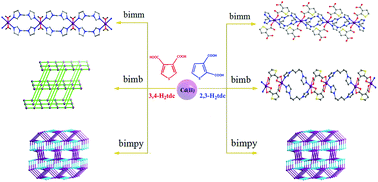
Two series of Cd(II) coordination polymers (CPs), {[Cd(bimm)2(H2O)2][(3,4-tdc)2][H2O]2}n (1a), [Cd(3,4-tdc)(bimb)]n (2a), [Cd(3,4-tdc)(bimpy)(H2O)]n (3a) and [Cd(2,3-Htdc)2(bimm)2]n (1b), {[Cd(2,3-tdc)(bimb)](H2O)}n (2b), [Cd(2,3-tdc)(bimpy)(H2O)]n (3b) where H2tdc = thiophenedicarboxylic acid, bimm = 1,2-bis(imidazol-1′-yl)methane, bimb = 1,2-bis(imidazol-1′-yl)butane and bimpy = 3,5-bis(imidazol-1′-yl)pyridine, have been synthesized by using Cd(II) acetate with H2tdc and N-donor ligands under hydrothermal conditions. Two related isomeric thiophenedicarboxylic acids were chosen to examine the positional isomeric effect on the construction of these CPs with distinct dimensionality and connectivity. The structure of 1a is a one-dimensional (1D) cationic double chain further forming a two-dimensional (2D) supramolecular network via hydrogen-bonding interactions, while 1b exhibits a neutral double chain structure. Interestingly, a three-dimensional (3D) 4-connected cds network for 2a as well as a 1D neutral double chain structure for 2b were obtained in the presence of bimb. When the rigid tripodal bimpy was introduced, isomorphous 3a and 3b with 3D (3,5)-connected (62·8) (67·83) nets were constructed. The structural diversity of 1a–2b mainly stems from the positional isomeric effect of thiophenedicarboxylate, while 3a and 3b are well regulated by rigid bimpy. Moreover, the thermal stability and photoluminescence of 1a–3b are investigated.

 Please wait while we load your content...
Please wait while we load your content...