Homoleptic and heteroleptic N-alkylimidazole zinc(ii)-containing ionic liquids for high current density electrodeposition†
Abstract
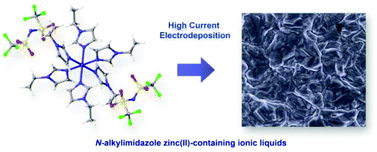
New homoleptic and heteroleptic zinc(II)-containing liquid metal salts with N-alkylimidazole (AlkIm) ligands and bis(trifluoromethylsulfonyl)imide (Tf2N−) anions are described. The general formulae of the complexes are [Zn(AlkIm)6][Tf2N]2 and [Zn(AlkIm)6−x(AlkIm′)x][Tf2N]2. Single-crystal X-ray diffraction revealed that, in the solid state, the cations consist of octahedral zinc(II) centres. The heteroleptic complexes contain two different N-alkylimidazole ligands. The melting points of the liquid metal salts are below or slightly above room temperature. The dependence of the melting points, viscosity and crystal structure on the alkyl chain length of the N-alkylimidazole ligand for the homoleptic complexes and on the ratio of the two N-alkylimidazole ligands AlkIm and AlkIm′ for the heteroleptic compounds is discussed. The possibility of incongruent melting and the presence of a mixture of the four-coordinate zinc(II) centre and neutral ligands is discussed. The new zinc(II)-containing liquid metal salts have been used as non-aqueous electrolytes for electrodeposition of zinc. A highly reversible deposition–stripping behaviour was found. Zinc electroplating was possible at very high current densities of more than −200 mA cm−2 in unstirred solutions. Compact and highly crystalline zinc deposits were obtained.

 Please wait while we load your content...
Please wait while we load your content...