Catalyst life in imidazolium-based ionic liquids for palladium-catalysed asymmetric allylic alkylation†
Abstract
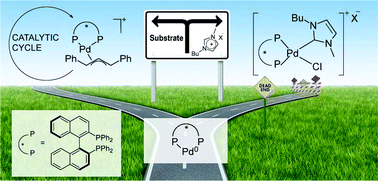
A Pd/(S)-BINAP system was successfully applied to the asymmetric allylic alkylation of rac-1,3-diphenyl-3-acetoxyprop-1-ene (I) using imidazolium-based ionic liquids (ILs) attaining up to 225 h−1 TOF and 88% ee of the (R)-product. Although the system was barely active in the recycling experiments, the catalyst life was confirmed after recharging the system with substrate/reactants resulting in an alkylated product. In the latter case, the conversion rates and enantiomeric excesses were similar or lower compared to those in the first cycle. In order to explain the observed catalyst performance in the recycling as well as in the recharging experiments, we investigated the reactivity between the catalyst precursors, substrate and reactants in ILs. We were able to identify the species involved in the catalytic reactions under various conditions by means of 31P NMR analyses. Allylpalladium intermediates (3) were found to be the active and selective species at a high substrate concentration. When the substrate was consumed, competing reactions took place leading to different palladium complexes. [PdCl(NHCBu,Me)((S)-BINAP)]Cl (4), together with [Pd((S)-BINAP)2] (5), were recognised as the species responsible for the loss of activity, meanwhile, the decrease in enantioselectivity was accounted for by the formation of mixed (NHC)(monophosphine)-palladium species.

 Please wait while we load your content...
Please wait while we load your content...