The various architectures and properties of a series of coordination polymers tuned by the central metals†
Abstract
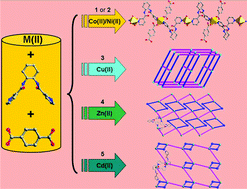
Five new metal–organic coordination polymers based on the mixed ligands of a semi-rigid bis-pyridyl-bis-amide N,N′-bis(3-pyridinecarboxamide)-1,2-cyclohexane (3-bpah) and 1,4-benzenedicarboxylic acid (1,4-H2BDC), namely [Co(3-bpah)(1,4-BDC)(H2O)3] (1), [Ni(3-bpah)(1,4-BDC)(H2O)3] (2), [Cu(3-bpah)(1,4-BDC)] (3), [Zn(3-bpah)(1,4-BDC)]·H2O (4) and [Cd(3-bpah)(1,4-BDC)(H2O)] (5), have been hydrothermally synthesized and structurally characterized. Complexes 1 and 2 are isostructural and display the similar 1D infinite chains, which are further linked via hydrogen-bonding interactions to generate 3D supramolecular frameworks. Complex 3 features a 3D polymeric framework with CdSO4-like topology. Complexes 4 and 5 show two similar 2D (2,4)-connected networks with (4·85)(4) topology, in which the 3-bpah ligands adopt different μ2-bridging coordination modes via the ligation of two pyridyl nitrogen atoms in 4 and via the ligation of one pyridyl nitrogen and one amide oxygen atom in 5. In addition, the central metals show different coordination geometries in 4 and 5. The adjacent layers of complexes 4 and 5 are finally extended into 3D supramolecular architectures through hydrogen-bonding interactions. The effects of the central metals on the structures and properties of complexes 1–5 have been discussed. The electrochemical properties of complexes 1–3 and fluorescent sensing behaviors of 4–5 toward ethanol and nitrobenzene have been investigated in detail.

 Please wait while we load your content...
Please wait while we load your content...