A novel thermal reduction method towards the synthesis and growth of two unlike morphologies of nickel nanostructures
Abstract
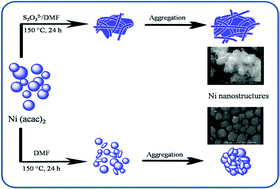
A facile method is described for synthesizing quasi-cotton fiber and spherical nickel nanostructures via a thermal reduction of Ni(acac)2 (acac = acetylacetonate) in N,N-dimethylformamide (DMF) and in the presence and absence of sodium thiosulfate, respectively. XPS analysis revealed the existence of Ni2+ and SO42− ions on the surface of nickel nanostructures obtained in the presence of anhydrous sodium thiosulfate. Magnetic measurements of this sample resulted in lower values of saturation magnetization (2.5 emu g−1) and remanent magnetization (0.6 emu g−1) and higher value of coercivity (206 Oe), compared to nickel nanostructure in the absence of sodium thiosulfate. The effects of the temperature, type of reactant and mole ratio of S2O32− : Ni2+ on the phase structure obtained products were demonstrated.

 Please wait while we load your content...
Please wait while we load your content...