Theoretical study on the stability of osmasilabenzynes†
Abstract
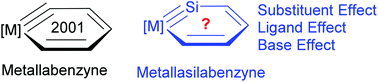
Metallabenzyne has attracted considerable interest from theoreticians and experimentalists since its first isolation in 2001. However, metallasilabenzyne, formed by the replacement of the carbyne carbon with a silicon atom in metallabenzyne, has never been reported either theoretically or experimentally. Here we carry out density functional theory (DFT) calculations on this system for the first time. Our results reveal a polarized and weak Os–Si triple bond in osmasilabenzyne due to the reluctance of the silicon to participate in π bonding. The effect of the ligands, substituents on the metallacycle, and bases on the stability or aromaticity of osmasilabenzyne is also discussed in detail. Specifically, an antibonding interaction between the metal and metal-bonded carbon and silicon in the HOMO of osmasilabenzyne is identified. Thus electron-donating substituents on the metallacycle can destabilize it. Because the Os–Si triple bond in osmasilabenzyne is highly polarized, a Lewis base can stabilize it by coordinating to the silicon atom. All these findings could be helpful for experimentalists to realize the first metallasilabenzyne.

 Please wait while we load your content...
Please wait while we load your content...