Fundamental chemistry of iodine. The reaction of di-iodine towards thiourea and its methyl-derivative: formation of aminothiazoles and aminothiadiazoles through dicationic disulfides†
Abstract
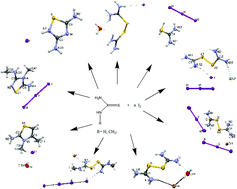
The reactivity of di-iodine towards thiourea (TU) and its derivative methylthiourea (MeTU) was studied. A diversity of products was obtained from these reactions. TU reacted with di-iodine in the absence or presence of hydroiodic or hydrochloric acids in a 1 : 1, 1 : 1 : 1 or 1 : 1 : 2 (TU : I2 : HX (X = I, Cl)) molar ratio to form the ionic compounds [(TU2)2+2(I−)·H2O] (1), [2(TU2) 2+·(Cl−)·2(I−)·(I3−)] (2) and [(TUH)+ (I3−)] (3). The compounds [(TU2)2+(Br−)(I3−)] (4) and [(TU2)2+2(Br−)·H2O] (5) were derived from the reactions of TU with di-iodine in the presence of hydrobromic acid in a 1 : 1 : 1 or 1 : 2 : 1 (TU : I2 : HBr) molar ratio. However, when the product of the reaction between TU and di-iodine in a 2 : 1 (TU : I2) molar ratio was crystallized in acetone–ethylether media the ionic salt of formula [(DAThdH+)(I−)] (6) (DAThd = 3,5-diamino-1,2,4-thiadiazole) was obtained. Methylthiourea (MeTU) reacted with di-iodine in the presence of hydrobromic acid (1 : 1 : 1, MeTU : I2 : HBr) in dichloromethane to form a solid product which gives [2(MeTU2) 2+·(2Br−)(I42−)] (7). Moreover, MeTU reacted with I2 in 2 : 1 (MeTU : I2) to form an intermediate powder product which was crystallized in acetone to give the 2-amino-3,4-dimethylthiazolium cation in [(DMeAThH+)(I−)(H2O)] (8). Upon changing the crystallization medium to ethanol, instead of acetone, the cationic 5-amino-3-methylamino-4-methyl-1,2,4-thiadiazolium (AMeAThdH)+ in [(AMeAThdH+)(I3−)] (9) was formed. The compounds were characterized by m.p., FT-IR, UV-Vis, 1H-NMR spectroscopy and mass spectrometry. The crystal structures of compounds 1–9 were determined by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...