Chiral rhodium complexes covalently anchored on carbon nanotubes for enantioselective hydrogenation†
Abstract
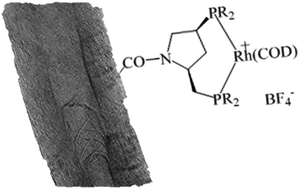
Chiral rhodium hybrid nanocatalysts have been prepared by covalent anchorage of pyrrolidine-based diphosphine ligands onto functionalized CNTs. This work constitutes the first attempt at covalent anchoring of homogeneous chiral catalysts on CNTs. The catalysts, prepared with two different chiral phosphines, were characterized by ICP, XPS, N2 adsorption and TEM, and have been tested in the asymmetric hydrogenation of two different substrates: methyl 2-acetamidoacrylate and α-acetamidocinnamic acid. The hybrid nanocatalysts have shown to be active and enantioselective in the hydrogenation of α-acetamidocinnamic acid. A good recyclability of the catalysts with low leaching and without loss of activity and enantioselectivity was observed.
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...