H/D exchange at sp3 carbons in the coordination sphere of platinum(ii)†
Abstract
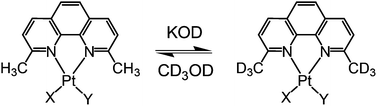
The [PtCl(η1-CH2CH2OR)(Me2phen)], Me2phen = 2,9-dimethyl-1,10-phenanthroline, complex is indefinitely stable in the solid state; however, when dissolved in protic deuterated solvents at basic pH, it undergoes H–D exchange at the Me2phen Me's. An analogous H–D exchange process takes place in the related [PtCl2(Me2phen)] complex which is sterically strained and very easily can detach one nitrogen of Me2phen yielding a T-shaped species. In contrast, the H–D exchange is considerably slower in the [Pt(OR)2(Me2phen)] complex characterized by smaller size and lower trans-labilizing effect of the oxygen donor ligands. It is suggested that the formation of a T-shaped intermediate could foster the C–H activation via oxidative addition to the metal centre. In accord with this hypothesis, the H/D exchange was found to be considerably slower in analogous complexes with 6,6′-dimethyl-2,2′-bipyridyl (Me2bpy), where the greater flexibility of Me2bpy, as compared to Me2phen, reduces the strain in the four coordinate substrate and hence the propensity to dissociate one end of the bidentate ligand.

 Please wait while we load your content...
Please wait while we load your content...