On the selective cleavage of nitrous oxide by metal–amide complexes†
Abstract
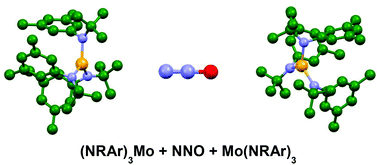
Computational investigation of nitrous oxide cleavage by metal–amide systems has shown that a bimetallic mechanism is compatible with the remarkable experimental observation of selective N–N bond scission by a di-molybdenum system, and that the interplay between the bonding energetics of reactants and products can both provide an explanation for the observed cleavage selectivity and be utilized to modify and design chemical behaviour.

 Please wait while we load your content...
Please wait while we load your content...