Synthesis, crystal structure and lithium ion conduction of Li3BP2O8†
Abstract
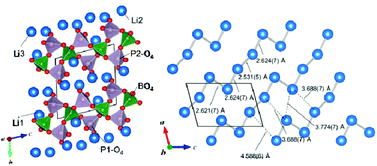
Single crystals of Li3BP2O8 were prepared by heating a mixture of starting materials with a Li : B : P molar ratio of 22 : 11 : 13 at 933 K in air and by cooling at a rate of −10 K h−1. The X-ray diffraction (XRD) reflections of a single crystal were indexed with triclinic cell parameters: a = 5.1888(5) Å, b = 7.4118(7) Å, c = 7.6735(7) Å, α = 101.18(1)°, β = 105.07(1)°, γ = 90.34(1)° (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2)). In the crystal structure of Li3BP2O8, BO4 and PO4 tetrahedra share O atoms and form one-dimensional 1∞[BP2O8]3− chains along the c-axis direction. A polycrystalline Li3BP2O8 bulk sample was synthesized by the solid state reaction of Li4P2O7 and LiPO3 prepared from the starting materials in advance with H3BO3 at 923 K. The lithium ion conductivities measured for the polycrystalline sample by the AC impedance and DC methods were 1.5 × 10−5 S cm−1 at 583 K and 6.0 × 10−8 S cm−1 at 423 K, respectively.
(no. 2)). In the crystal structure of Li3BP2O8, BO4 and PO4 tetrahedra share O atoms and form one-dimensional 1∞[BP2O8]3− chains along the c-axis direction. A polycrystalline Li3BP2O8 bulk sample was synthesized by the solid state reaction of Li4P2O7 and LiPO3 prepared from the starting materials in advance with H3BO3 at 923 K. The lithium ion conductivities measured for the polycrystalline sample by the AC impedance and DC methods were 1.5 × 10−5 S cm−1 at 583 K and 6.0 × 10−8 S cm−1 at 423 K, respectively.

 Please wait while we load your content...
Please wait while we load your content...